
News and Announcements from the CDB
The skin tissue is a complex organ system comprising multiple organs. In addition to the epithelium, dermis and subcutaneous fat—the three tissue layers making up the bulk of the skin—it includes appendage organs such as sweat glands, sebaceous glands and hair follicles. These organs are all connected to structures such as sensory nerves or muscles, and work together to produce physiological responses. Many scientists have been working to develop artificial skin that closely resembles the function and structure of natural skin for therapeutic use to restore skin function in patients suffering from different skin-related diseases or injuries and for use in testing the safety or effects of cosmetics or drugs. Thus far, it has been possible to induce pluripotent stem cells to differentiate into specific cell types of the skin, but generating a three-dimensional (3D) skin structure complete with appendage organs has proven to be difficult.
Now, a research team headed by Takashi Tsuji, Team Leader of the Laboratory for Organ Regeneration, has reported the successful generation of skin tissue complete with appendage organs such as hair follicles and sebaceous glands from mouse induced pluripotent stem cells (iPSCs). They developed a new approach to efficiently induce generation of 3D skin tissues from iPSCs by transplanting them into an in vivo system. Furthermore, they demonstrated that their derived skin tissue was fully functional when implanted into the skin of a nude mouse, with transplanted skin engrafting with the host tissue and its hair follicles exhibiting regular hair eruption cycles similar to that of natural skin. This work was published in the online journal Science Advances.
Generally, the transplantation of iPSCs or embryonic stem cells into an in vivo system leads to the formation of teratomas, a tumor consisting of a mix of tissue types derived from the three embryonic germ layers. Tsuji’s group began by searching for conditions favorable for inducing the formation of epithelial tissues, which is essential for organogenesis, from iPSCs at high efficiencies. They developed a novel method to transplant numerous embryoid bodies (EBs) formed from culturing iPSCs clustered in drop of collagen gel into a live mouse, calling this the clustering-dependent embryoid body (CDB) transplantation method. The team found that transplantation of clustered EBs in collagen drop produced four times as many cysts containing epithelial tissues than observed from transplantation of single iPSCs or EBs. These cystic epithelia possessed the three epithelial tissue layers as well as hair follicles and sebaceous glands that make up whole skin tissue all arranged akin to that of natural skin. They also discovered that stimulating EBs with Wnt10b, signaling factor important for hair follicle formation, markedly increased the number of hair follicles formed within cystic epithelia.
Next, the team cut out small units of skin tissue with hair follicles from the cystic epithelia, implanted them subcutaneously into the back skin of a mouse. To trace engraftment of the transplanted skin unit, skin tissue made from male mouse-derived iPSCs was transplanted into a female mouse, and using Y-FISH analysis, which labels Y-chromosome positive cells, they verified that their iPSC-derived skin engrafted with the surrounding host tissue and showed no signs of tumorigenesis. Close analysis of the transplanted skin tissue revealed that it formed proper connections to nerves, muscles, and other structures of the host, and the hair follicles themselves underwent repeated hair cycles consistent with natural skin. The color, shape, type, pattern and density of the erupted hair was also confirmed to be similar to that of normal mice skin.
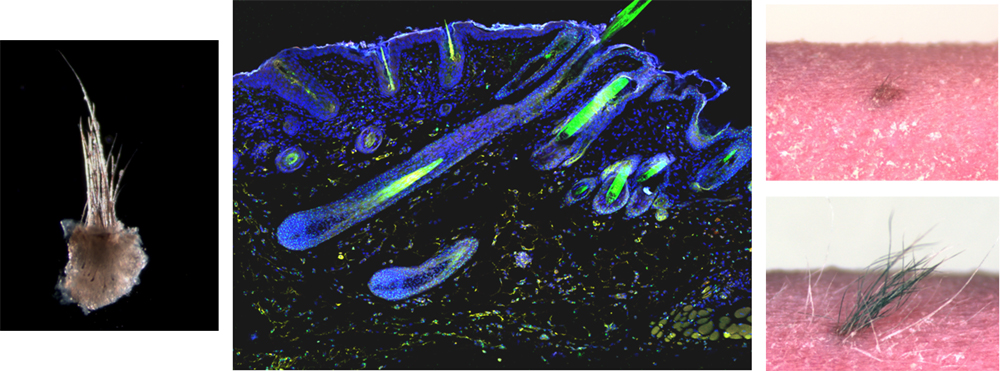
Skin tissue units including hair follicles (left) from iPSC-derived cystic epithelia were excised and transplanted subcutaneously in a nude
mouse. Transplanted tissue unit became engrafted in host tissue (middle: explant-derived Y-chromosome-positive cells (green) and
nucleus (blue)) and displayed normal repeating hair cycles (right).
“Our CDB method appears to induce complex skin tissue through the interactions between outer epithelial surfaces of neighboring EBs, which may be creating epithelial niches with increased surface areas that undergo epithelial-mesenchymal interactions needed for skin organogenesis,” says Tsuji. “The finding that Wnt10 signaling increases induction frequency of hair follicles provides an important hint for future studies attempting to replicate skin organogenesis in a culture system. We aim to understand the basic principles at work during organ induction as well as strive to develop artificial skin that can be used for human clinical applications and in industry.”
| Link to article |
|---|