
News and Announcements from the CDB
Pulmonary alveoli are small, spongy, sac-like structures found at the terminal ends of the branching respiratory system and is the region of the lung where gas exchange takes place. They comprise the planar type I alveolar epithelial cells involved in gas exchange, the round type II alveolar epithelial cells that secrete surfactants to regulate humidity inside alveolar sacs, and the myofibroblasts that form the septa between alveolar ducts and help maintain the lung structure, which undergoes significant expansion and contraction during respiration. Alveolar development (alveologenesis) is unique in that it spans both embryonic and postnatal stages of development, and the distinctive morphology of the alveoli arises postnatally following the initial contact with air inhaled through respiration. The molecular mechanisms underlying alveologenesis, especially those involved postnatally, however, remain largely unknown.
Now a new study by technical staff Chisa Matsuoka and Team Leader Mitsuru Morimoto of CDB’s Laboratory for Lung Development, published in the Proceedings of the National Academy of Sciences USA, demonstrates the critical role of Notch signaling in postnatal alveolar development using the mouse as a model. They show that disruption of Notch signaling, Notch2 in particular, in epithelial cells lining the airways leads to abnormal alveolar morphology reminiscent of the phenotypes of bronchopulmonary dysplasia, with enlarged alveolar airspaces and decreased cellularity, reducing efficiency of gas exchange. This study was carried out in collaboration with National Taiwan University and Columbia University (U.S.A).
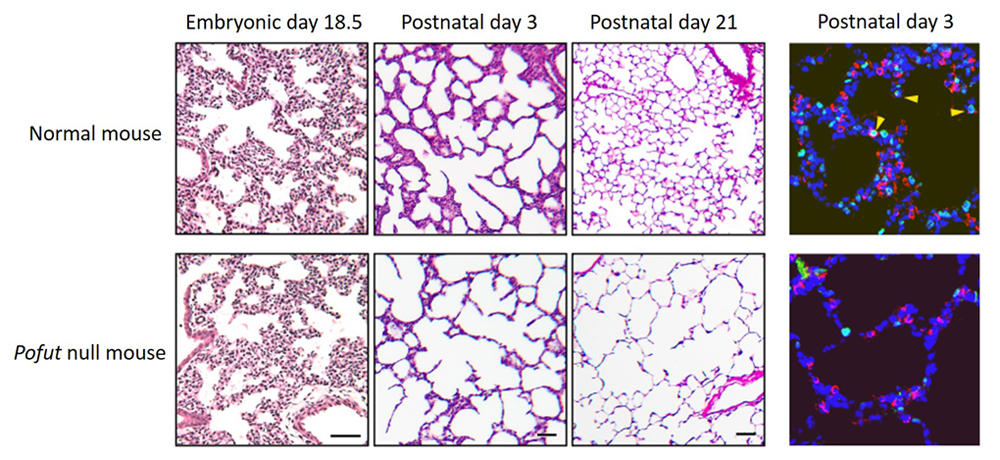
The Notch signaling pathway is important in lung morphogenesis, particularly in the differentiation of bronchial epithelial cells (see Science news: Jan. 29, 2016 and Dec. 13, 2012), and mice lacking epithelial Notch die shortly after birth, making it difficult to study the role of Notch in postnatal alveolar development. Thus, to carry out a more detailed analysis of alveolar development, the research team examined a Notch-deficient mouse line which shows moderate lethality, as well as a few knockout mouse lines for Notch signaling-related genes that partially impair Notch function. Analysis of a Pofut1 knockout mouse line which leads to inactivation of epithelial Notch signals revealed that progression of prenatal pulmonary development is relatively normal, but abnormalities arise postnatally during alveologenesis. The lungs of Pofut1 mutants were reminiscent of the emphysema-like phenotype with enlarged airspaces in the alveolar structure. Notch2 was discovered to be the critical signal required for postnatal alveologenesis, with the lungs of Notch2 mutants displaying significantly reduced numbers of type II cells in the alveoli. The team determined that a drop in proliferation of type II cells in Notch2 mutants that may lead to overall reduction numbers of type II cells seen in alveoli, as type II cells serve as progenitors to generate both type I and type II cells.
Notch-deficient mice also exhibited abnormalities in the mesenchymal cells of alveoli in addition to the epithelial cells. During postnatal alveolar development, the alveolar septa begin to form from the mesenchymal myofibroblast cells lining the pulmonary epithelium, increasing pulmonary surface area for gas exchange. In the Notch2-deficient mouse, the number of myofibroblast cells found in the distal respiratory branches were reduced which in turn resulted in fewer septa formation. These myofibroblast cells are known to be activated to proliferate through PDGF-A, a growth factor secreted by the epithelial cells and known as a downstream factor of Notch signaling. PDGF-A expression was indeed reduced in Notch2-defective epithelium. Thus, loss of Notch signals disrupted epithelial-mesenchymal interactions, causing severe abnormalities in alveolar morphogenesis.
“Our present study focused on developmental events occurring postnatally, rather than those seen prenatally, and has opened up new avenues of research. Emphysema is caused not only by impaired alveolar development, but can arise during adulthood triggered by external factors or from aging. Thus, understanding postnatal lung morphogenesis may help us to discover ways to regenerate damaged lung tissues,” explained Morimoto. “There are many factors at work in an in vivo system that make it difficult to gain clear insights into normal developmental processes. Our group is now working to develop an in vitro system to recapitulate pulmonary development, which would allow us to clue into the key mechanisms required for this process.”