
News and Announcements from the CDB
The cerebellum is a vital region of the brain intimately involved in the regulation of motor coordination and some aspects of cognition. Disorders affecting the cerebellum can be debilitating, leading to loss of speech articulation and uncoordinated and unsteady gait. One group of hereditary genetic diseases linked to the cerebellum is spinocerebellar ataxia (SCA), an intractable disorder characterized by progressive loss of motor coordination, while the patients retain full mental capacity. There are numerous types of SCA and many causative genes have been identified, but much remains unknown about underlying molecular mechanisms of SCA pathogenesis and no effective treatments are currently available for this disease. Thus, there is a need for a suitable model system to study SCA pathogenesis and to screen for drug compounds that may be effective for treating SCA.
Now, a study by CDB visiting scientist Yoshihito Ishida*1 and research specialist Keiko Muguruma of the Laboratory for Cell Asymmetry reports the successful generation of mature Purkinje cells from induced pluripotent stem cells (iPSCs) derived from SCA type 6 (SCA6) patients. They further reveal that when placed under conditions of stress, the patient-derived Purkinje cells exhibit some vulnerabilities, and demonstrate that this stress culturing method can be used as an in vitro model system for evaluating drug candidates for treating SCA. Their work, published in the journal Cell Reports, was carried out in collaboration with Hiroshima University and Kyoto University.
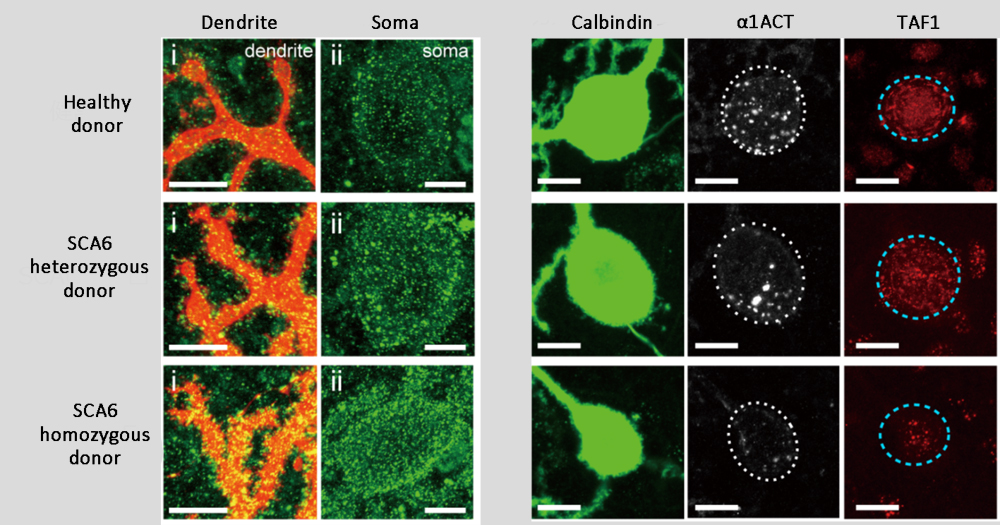
SCA6 is characterized by selective degeneration and loss of Purkinje cells in the cerebellum, and cerebellar atrophy. It is caused by a mutation in the CACNA1A gene encoding the Cav2.1, the α1-subunit of a calcium channel. The mutated gene has an expanded CAG trinucleotide repeat in the region encoding the Cav2.1 C-terminal domain, resulting in an abnormally long section of glutamine residues (or polyQ). Muguruma, who previously reported the successful generation of mature Purkinje cells from human embryonic stem cells and iPSCs (see Science News: March 2, 2015), and her collaborators wondered whether it would be possible simulate pathology of SCA6 in vitro by applying their protocol for Purkinje cell differentiation using patient-derived iPSCs.
The team generated iPSCs from skin or blood cells of healthy donors and of SCA6 patients with heterozygous alleles or homozygous alleles for CAG repeats, and confirmed that their established iPSC lines have the same number of CAG repeats as their respective donors. The iPSCs were then differentiated into Purkinje cells, and similar to known SCA pathology, patient-derived Purkinje cells showed high cytoplasmic levels of Cav2.1. A fragment of the C-terminal domain of Cav2.1 containing the CAG repeat, called α1ACT, also functions as a transcription factor targeting TAF1 in the nucleus, which is involved in cell growth and development. Expression levels of α1ACT and TAF1 were found be to markedly reduced in patient-derived Purkinje cells than in control Purkinje cells, which was consistent with reports from other studies indicating that α1ACT containing CAG repeat expansion loses ability to activate transcription of TAF1 and consequently inhibits neural cell growth and development.
Recapitulating in vivo disease pathology in a cell culture system within a relatively short period presented a challenge as SCA6 is a late-onset disease, meaning symptoms begin appearing later in life. Thyroid hormone T3 is known to be an essential factor for Purkinje cell maturation and maintenance, the group temporarily depleted T3 from their culture system to create conditions of stress and analyzed its effect on Purkinje cells. Whereas Purkinje cells from healthy donors showed no significant changes, many of the patient-derived Purkinje cells died or if they did survive, had significantly reduced dendritic arborization, indicating that T3 depletion caused vulnerability in patient-derived Purkinje cells.
The group also explored the possibility of using their patient-derived Purkinje cells following stress for drug assays, testing different compounds on their effectiveness in reducing the vulnerabilities observed in patient-derived Purkinje cells. They found that thyroid releasing hormone (TRH), which has been used in treatments for SCA6, and a compound called Riluzole, which has been used in treatments for another neurodegenerative disorder called ALS, both displayed positive effects in improving survival and maintenance of patient-derived Purkinje cells.
“The strength of our in vitro model comes from combining the techniques for generating human iPSCs from patients with those for inducing self-organizing cerebellar tissues and Purkinje cells to recapitulate ontogenesis,” explains Muguruma. “Other types of SCA involve degeneration of other neural cells in the cerebellum as well as in the cerebrum. With our expertise in generating cerebellar and cerebral tissues from disease-specific iPSCs, we hope to contribute to unveiling the pathology of different SCA types and other neurodegenerative diseases.”
*1 Visiting scientist Yoshihito Ishida is employed by Shionogi & Co. Ltd., however the company was not directly involved in any aspect of this study.
| Link to article |
Vulnerability of Purkinje Cells Generated from Spinocerebellar Ataxia Type 6 Patient-Derived iPSCs. |
|---|---|
| Related link |
Generating 3D cerebellar structure and functional cerebellar neurons |