
News and Announcements from the CDB
The neocortex is a six-layered brain region unique to mammals and is made up of a complex network of multiple cell types. This region is particularly large in primates, including humans, and is associated with higher order brain functions such as perception, thinking, memory, and language. Neurons of the neocortex are derived from progenitor cells that lie in the ventricular zone, and these progenitors divide and differentiate to generate deep-layer (DL) projection neurons and then upper-layer (UL) projection neurons in a timely manner, with the later-born neurons migrating to upper layers. The transition from generating one neuronal subtype to another is critical for creating the layered organization of the neocortex, but the mechanisms involved in this regulation are still shrouded in mystery.
Now, a study by research scientist Kenichi Toma of the Laboratory for Neocortical Development (Carina Hanashima, Team Leader) and colleagues, published in the Journal of Neuroscience, identifies the cues involved in triggering the onset of UL neurogenesis in the developing neocortex. Using a series of transgenic mouse lines, they found that induction of UL neurogenesis requires sequential derepression of transcription factors, triggered by Foxg1, and also a feedback signal from earlier-born, differentiated DL neurons. Thus, the neural progenitors use a combination of intrinsic gene regulatory mechanisms and extrinsic cues from surrounding cells to switch from DL to UL neurogenesis.
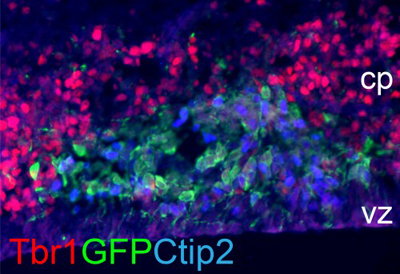
Coelectroporation of Foxg1 and GFP constructs (shown in green) in brain
cells of Foxg1 knockout mouse, represses Tbr1 expression (red) and triggers
the expression of DL neuron marker Ctip2 (blue).
In neocortical development, Cajal-Retzius (CR) cells, which occupy the most superficial layer of the cortex, are generated first, followed by DL neurons that are generated from embryonic day (E)11.5, and UL neurons born at E14.5. These neurons then undergo a process of maturation to form a six-layered structure. Previous work from the laboratory has shown that expression of a transcription factor, Foxg1, triggers progenitors to enter DL neurogenesis from CR cell production (see CDB News: April 5, 2013). The regulatory mechanisms involved in the switch from DL neurogenesis to UL neurogenesis, however, remained unclear.
In the current study, the team examined development of UL neurons in detail using transgenic mouse lines previously developed in the lab, in which timing of Foxg1 expression could be manipulated. Under normal conditions, the expression of this gene first appears around E8.5. When they delayed the expression onset to a later developmental stage (at E14.5), the period of CR neuron generation was prolonged concomitantly, until the expression of Foxg1 rapidly induced DL neurogenesis. Using different markers to label the cohort of neurons produced at E14.5 and those produced the next day, the team found that at E18.5 most of the earlier-born neurons had differentiated into DL neurons, while the later-born neurons had primarily differentiated into UL neurons. The further 1-day delay of Foxg1 induction to E15.5 also gave similar results, suggesting that irrespective of the progression of development, the onset of UL neurogenesis cannot bypass DL neurogenesis.
Maintaining the layered structure of the neocortex also requires regulation of transcription factors. Each differentiated layer subtype expresses specific transcription factors, and is segregated from the other layer subtypes through mutual repression of four transcription factors. However, how this repression network is triggered to confer fate specification in neurogenesis was not well understood. Toma et al. speculated that Foxg1 was the trigger to tip the balance, and looked into the gene expression levels of the four transcription factors after Foxg1 was induced. Tbr1 was the only transcription factor out of the four that showed a marked decrease in expression levels after Foxg1 induction. The cells in which Tbr1 was suppressed after Foxg1 induction also showed a rise in expression of DL neuron marker, and went on to differentiate into DL neurons, indicating that induction of Foxg1 leads to suppression of Tbr1 in progenitor cells signaling them to commit to DL neuronal fate.
So after induction of DL neurogenesis, how do the progenitors time the switch over to UL neurogenesis? Under culture conditions, progenitors need to be maintained under relatively high density conditions to induce differentiation of UL neurons. Fixing on this point, the team examined whether onset of UL neurogenesis could be regulated by an extrinsic signal originating from DL neurons themselves. They generated a mouse line in which DL neurons produced between E11.5 to E13.5 were specifically ablated, and found that, despite a large drop in DL neuronal population immediately after ablation, their numbers had recovered substantially by E18.5. UL neurons were also generated, although slightly fewer than seen under normal conditions. Closer examination revealed a prolonged period of DL neurogenesis, which in turn led to a delay in the onset of UL neurogenesis. These results indicate that the differentiated DL neurons send a signal to the progenitors to halt DL neurogenesis and initiate UL neurogenesis.
“The mechanisms uncovered in this study are extremely useful systems for generating the necessary neuronal subtypes in sequential order, as well as maintaining the relative balance of their number regardless of the size differences, for example, between mouse and human brains,” says Hanashima. “Our next step is to reveal what molecular signal from DL neurons is relayed to progenitors to facilitate the switch to UL neurogenesis, bringing us closer to understanding the mechanisms of neocortical neurogenesis.”
| Link to article | |
|---|---|
| Related link |
Transcriptional switch controls neuronal fate in neocortex(Apr 5, 2013) |