
News and Announcements from the CDB
During development of the retina, a section of the neuroepithelium balloons outwards to form a sac-like optic vesicle, which then folds inwards to produce a two-layered optic cup. The cells in the outer layer of the optic cup differentiate into the retinal pigment epithelium (RPE), while those in the inner layer differentiate into the neural retina which includes the light-sensing photoreceptor cells. At the peripheral margin of the embryonic retina lies the ciliary margin (CM), the junctional region where the neural retina and the RPE converge. In birds, fishes, and some reptiles, the CM is known to maintain a stem cell population that contributes to retinal development and regeneration. Whether or not a similar stem cell population exists in the CM of the mammalian eye is unclear, and in humans, where access to the embryonic retina is difficult, even less is known about this region.
Now, a new study carried out by Atsushi Kuwahara, a visiting scientist in the Laboratory for Organogenesis and Neurogenesis (Masatoshi Takeichi, team leader; laboratory closed in March 2015), Mototsugu Eiraku, team leader of the Laboratory for In vitro Histogenesis, and other colleagues shows evidence that strongly suggests the CM of the embryonic human retina also fosters a stem cell population which plays a key role in the growth of the retina. The research team developed a protocol allowing them to drive human embryonic stem cells (ESCs) to spontaneously self-organize to generate a three-dimensional (3D) retinal structure that includes CM-like regions at high efficiencies. They then found that the CM-like region is a niche for retinal stem cells and serve as a source of progenitors to produce new neural retinal cells. This work, published in Nature Communications, was a joint project with the Environmental Health Science Laboratory, Sumitomo Chemical Company Ltd.
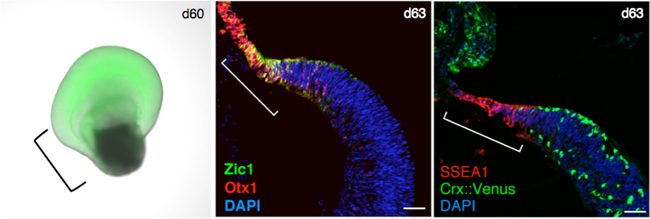
The Laboratory for Organogenesis and Neurogenesis, under the direction of former group director Yoshiki Sasai, previously developed the SFEBq (Serum-free Floating culture of Embryoid Body-like aggregates with quick reaggregation) method, a 3D cell culturing method that can efficiently induce differentiation of pluripotent stem cells to form a neuroepithelial sheet. With the SFEBq method, the ES or iPS cells initially form floating aggregates, which then differentiate into neural progenitors, and the subsequent addition of different cocktails of signaling factors stimulates spontaneous self-organization of these cells to further differentiate and produce 3D neural tissues, partially recapitulating embryonic developmental processes seen in vivo. To date the laboratory has been able to induce the generation of tissues resembling cerebrum, hypothalamus, pituitary gland, and cerebellum. They also successfully generated 3D stratified retinal tissue from both mouse and human ESCs (see CDB News: April 7, 2011; June 14, 2012), which showed the formation of a region at the boundary between the neural retina and RPE containing cells distinct from both cell types. The research team used this as their starting point to determine whether CM could indeed be generated in hESC-derived retinal tissue, and to uncover the role of the CM.
Kuwahara et al. first focused on improving the induction efficiency of the initial differentiation step from ES cells to retinal tissues. They found that the transient addition of Bone morphogenetic protein-4 (BMP4) to the culture system when differentiation begins could drive ES cells to differentiate into retinal structures at markedly higher efficiencies and with greater stability, and called this improved differentiation method, the BMP method. This method also eliminated the need to add extracellular matrix proteins to the culture media—the addition of which poses difficulties when considering large scale production and future clinical applications. Analyses of the retinal tissues generated with this method showed that most had gene expression profiles characteristic of neural retina, and not RPE.
The researchers next speculated that if they could produce both neural retina and RPE inside cultured cell aggregates, it might be possible to generate a CM, which in normal development, forms at the junction between the neural retina and the RPE. Reports from past studies have indicated the importance of Wnt signals for RPE differentiation as well as maintenance, and FGF signals for neural retina differentiation and maintenance. Focusing on these two signaling pathways, the team attempted to create a two-domain retinal tissue that includes both the neural retina and RPE. They tested a range of conditions, and established a protocol which could generate the said retinal tissue with high efficiency and reproducibility; BMP-method derived retinal tissue (at differentiation day 18) is treated for six days with Wnt agonists and FGF inhibitors to tip the differentiation bias toward RPE cell fate, and then placed again under conditions favorable for inducing neural retina. Interestingly, immunostaining and live imaging analyses of the derived tissue indicated that the tissue which was initially biased toward a neural retina fate, was transiently biased to an RPE fate after treatment with Wnt agonists and FGF inhibitors, before resuming a neural retina fate. They called this method the ‘induction-reversal’ method because of the swinging of fates of the cell aggregate between neural retina and RPE.
When the retinal tissue generated with the induction-reversal culture was cultured over a two-month period, the neural retina and RPE both continued to grow, with the tissue eventually taking on a turnip-shaped form. The junctional region between the neural retina and the RPE of the derived retinal structure was distinctly thin and tapered in appearance, and expressed genes specific to the CM. Following three months of culture, the neural retina comprised numerous photoreceptor progenitors, and by five months, there were differentiated photoreceptor cells present. When the tapered CM-like region was examined, they found that there was a large number of stem cells with high sphere-forming abilities, serving as a source of progenitors for the growing retinal tissues.
“Our results are consistent with the current view that the RPE and neural retina are capable of fate transition. We hope to further unveil the mechanisms involved human retinal development through examining retinal formation in culture,” says Eiraku.
Lead author Kuwahara adds, “The protocol developed here allows us to generate retinal tissue that more closely resembles the biological retina at higher efficiencies and with greater stability. It is a step closer to realizing regenerative medicine for retinal disorders, and we hope to continue advancing our research in this regard.”
| Link to article |
Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue |
|---|---|
| Related link |
The self-made eye: Formation of optic cup from ES cells(April 7, 2011) |
| Related link |
Self-organized retinal tissue from human embryonic stem cells(June 14, 2012) |