
News and Announcements from the CDB
The germ cell, more commonly referred to as oocyte or sperm, are the only cell type in the animal body that contributes to the next generation by passing on its genetic information. Despite its importance in reproduction, oocytes have been found to display a surprisingly high rate of chromosome segregation errors as maternal age increases. When an oocyte with abnormal chromosomal numbers (aneuploid) is fertilized, it often results in a miscarriage or results in congenital disorders such as Down Syndrome if it develops to term. While chromosome segregation errors are known to occur during the first meiotic division (MI) of oocytes, the exact causes and mechanisms remain unclear.
A new study published in Nature Communications by Yogo Sakakibara, a special postdoctoral researcher in the Laboratory for Chromosome Segregation (Tomoya Kitajima, team leader) and collaborators at the IVF Namba Clinic, Japan, and the Karolinska Institute, Sweden, takes a closer look at the chromosome dynamics during MI in both mouse and human oocytes. Using high-resolution 3D imaging microscopy to track chromosome movement, they demonstrate that premature separation of bivalent chromosomes during MI is a major cause of chromosome segregation errors observed in aging oocytes.
Movie: Chromosome dynamics during MI in an oocyte from 16-month-old mouse (blue, chromosomes; green, kinetochores). Chromosome localization was traced by tracking movements of kinetochores (section of chromosome that bind to microtubules). One of the bivalents was hyperstretched by microtubule-generated bipolar forces and eventually separated prematurely into univalents. Univalents then underwent predivision of sister chromatids (movie shows balanced predivision).
Germ cells arise from meiosis, a specialized type of cell division which reduces the chromosome number by half through two rounds of cell division, meiosis I (MI) and meiosis II (MII). During MI, maternal and paternal chromosomes pair and undergo synapsis to form homologous chromosome sets (bivalents), and these bivalents then separate into univalents and are evenly divided into two daughter cells. In MII, sister chromatids of univalents are separated and are again evenly divided into two cells, resulting in haploid cells. A study published by Henderson and Edwards in 1968 reported the observation of a high frequency of univalents in chromosome spreads taken from aged oocytes undergoing MI, and proposed that this was likely the cause of chromosome segregation errors. Many studies following their work appeared to support this hypothesis; however, it remained difficult to prove directly due to small sample sizes and limitations in experimental methods.
In the present study, Sakakibara and his collaborators successfully used high-resolution 3D imaging technology to examine and analyze chromosome dynamics in live oocytes during MI. They began by analyzing 275 oocytes obtained from naturally aged mice (16 months), and found that 20 of those oocytes exhibited errors in chromosome segregation. They identified three distinct patterns of segregation errors. The first was balanced predivision (45%), in which both pairs of sister chromatids making up a bivalent are separated prior to MII. The second was unbalanced predivision (35%), in which one pair of the sister chromatids of a bivalent is segregated prematurely. In the third pattern, both pairs of sister chromatids fail to separate, resulting in nondisjunction (20%). In cases of balanced predivision, the oocyte appeared as though normal chromosomal segregation has taken place; however, errors will arise during the second meiotic division. In unbalanced predivision and nondisjunction, the oocyte contains odd chromosome numbers, whereas in cases of nondisjunction, they have an even chromosome numbers. These patterns of segregation errors in the mouse, are consistent with reports from human genetic studies and analysis of aneuploidy in human oocytes. Chromosome segregation errors were not observed in the 167 oocytes obtained from young mice (two months old).
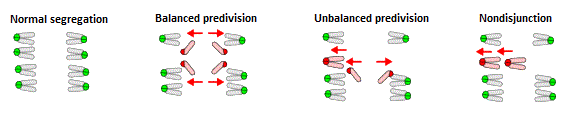
Three patterns of segregation errors observed in oocytes from naturally aged mouse (16 months) during MI.
Next, the group re-examined the chromosome dynamics to determine the underlying cause of these segregation errors. Univalents were found in 80% of oocytes exhibiting segregation errors during a period of MI when bivalents should be found. The team discovered that while bivalents need to maintain their structure until the segregation occurs in MI, some are unable to withstand the bipolar forces generated by the microtubules and consequently become hyperstretched until they eventually prematurely separate into univalents. The same bipolar forces bioriented the univalents, and in many cases, the sister chromatids of the univalents were mistakenly segregated in MI. These findings suggest that the glue holding the bivalent together until segregation is weakened, corresponding with recent reports of reduced levels of cohesin, a protein complex mediating cohesion between sister chromatids of bivalents, in aged oocytes.
Sakakibara and his colleagues also analyzed chromosome segregation dynamics in human oocytes. They obtained oocytes that would otherwise be disposed of from a fertility clinic, with the donors’ consent, and found that, similar to the results observed in mice, oocytes from relatively aged donors exhibited premature bivalent chromosome separation during MI. The resulting univalents were then pulled to opposite poles, strongly suggesting that premature segregation of bivalents is a cause of chromosomal segregation errors seen in humans.
“While our study clearly demonstrated the major cause of age-related chromosomal segregation errors observed in the mouse, we need to be careful when trying to interpret the data from human oocytes,” explains Kitajima. “We are planning to examine the process of premature bivalent separation from a molecular perspective. In particular, we hope to reveal the mechanism underlying the reduction of cohesin in aged oocytes.”
| Link to article |
Bivalent separation into univalents precedes age-related meiosis I errors in oocytes |
|---|