
News and Announcements from the CDB
Olfaction or sense of smell, while not critical for life, can greatly influence the quality of life. The olfactory system is capable of detecting and discriminating a wide range of airborne odor molecules in the air; odorants inspired into the nasal cavity contact odorant receptors (ORs) expressed by olfactory sensory neurons (OSNs) of the olfactory epithelium, and the OSNs then relay the information via the glomeruli in the olfactory bulb (OB) to the mitral and tufted cells, the second order neurons in the olfactory system. While the olfactory neural circuits are stably maintained throughout life, damage to OSN axons due to head trauma in adults can lead to an olfactory disorder called dysosmia, where patients experience reduced odor sensitivity as well as unpleasant perception of different odors. It has yet to be shown how the neural circuitry is affected after OSN axon injury, which may yield hints for ways to treat dysosmia.
New work by junior research associate Aya Murai and others in the Laboratory for Sensory Circuit Formation (Takeshi Imai, Team Leader), in collaboration with Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, carried out a detailed analysis of a mouse model for dysosmia, and discovered that during the recovery process following axonal injury, OSN axonal targeting was impaired and the connectivity between axons of OSNs and dendrites of mitral cells was also markedly reduced. Their study, published in the online journal eNeuro, unveils the mechanism underlying dysosmia pathogenesis.
The neural circuitry of the olfactory system is elaborate and complex. Each OSN expresses a single OR type, and OSNs expressing the same ORs all converge their axons to one glomerulus in the OB. Olfactory information is interpreted from the activation patterns of over 1,000 sets of glomeruli, stimulated by over 1,000 types of OSNs. Unlike other neurons, OSNs are unique in that they can be regenerated, and undergo continuous turnover throughout life, while maintaining the original topographical circuitry. After damage to OSN axons by head trauma, newly generated OSNs often show impaired axonal connections, but why this occurred was not well understood.
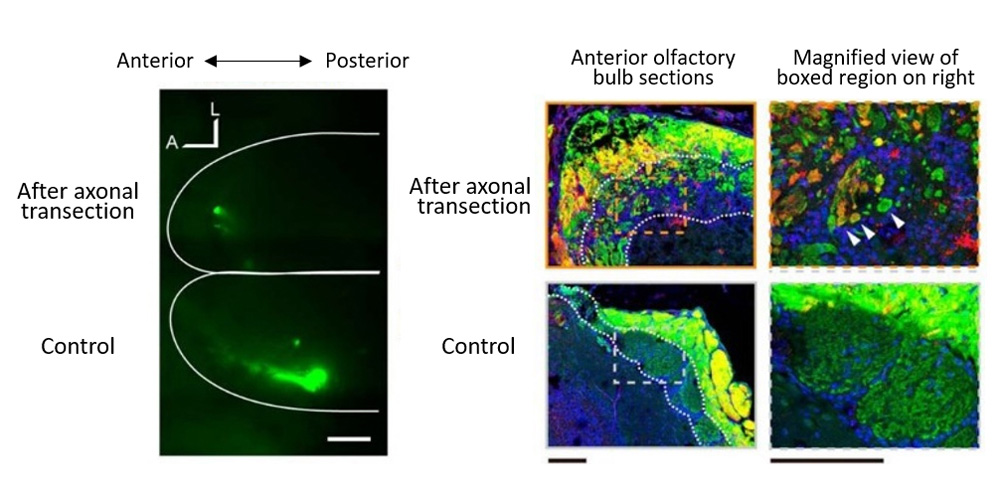
The team surgically transected a portion of OSN axons projecting to the olfactory bulb to generate a mouse model simulating axonal injury following head trauma, and then examined the recovery process of the OSN, searching for the mechanism underlying dysosmia. Normal OSN turnover takes place in two phases; first, OSN roughly determine the anteroposterior course to project their axons, and then as axonal projection progresses, will refine their path as needed to their glomerulus target. In contrast, in the axonal injury mouse model, the initial anteroposterior course targeting of axons was often impaired, with axons extending a direction opposite to that seen in normal OSN turnover. Their experiments also revealed a concentration of mistargeted axons in more anterior region of olfactory bulb and formation of small glomerulus-like structures innervated by heterogeneous mix of OSN axons.
They also analyzed the mitral cells, which relay signals received from the glomerulus to the olfactory cortex. Mitral cells are normally connected to the glomerulus through a single primary dendrite, but after OSN axonal injury, only half of the mitral cells were found to maintain connections with OSNs, and the arborized tips of the primary dendrites showed signs of atrophy contributing to reduction in connectivity. Live imaging in vivo of mitral cell activity also confirmed that axonal injury model showed reduced olfactory sensitivity than normal mouse.
“When OSN axons are severed due to severe head trauma, we saw impaired targeting of axons as well as reduced connectivity to mitral cells. This suggests that in normal OSN turnover, the existing OSN axons act as part guide and part scaffold to direct new OSN axons to the correct target,” explains Imai. “Thus, degeneration and eventual loss of ‘scaffold’ axons after injury appears to be the underlying cause of dysosmia. It may be possible to prevent dysosmia if OSN axon degeneration after injury can be delayed enough to allow newly generating OSNs axons to be guided to their correct targets. We would like to continue seeking hints to treat dysosmia.”
| Link to article |
|---|