
News and Announcements from the CDB
The aroma of freshly baked cookies wafts past your nose. The source of the smell is a mixture of aromatic compounds or odorants floating in the air. Each odorant making up the aroma of freshly baked cookies will bind to and stimulate a specific neuron, called the olfactory sensory neuron (OSN), in the nasal cavity. Each OSN expresses a single type of odorant receptor; humans possess approximately 400 different types of OSNs, while mice have approximately 1,000 types. Regardless of closeness to the source of the smell or intensity of the smell, we are capable of identifying a specific smell from amongst a huge variety. The robustness in our ability to discriminate odors independent from odor concentration, has long remained unresolved.
In a study led by CDB visiting researcher Ryo Iwata of the Laboratory for Sensory Circuit Formation (Takeshi Imai, Team Leader), published in the journal Neuron, they examined the neuronal firing patterns in the mouse olfactory bulb, specifically the mitral cells, when stimulated by odors. Using a two-photon calcium imaging system to visualize neuronal activity, they reveal that odorants are identified by differences in temporal firing of mitral cells in the glomerulus.
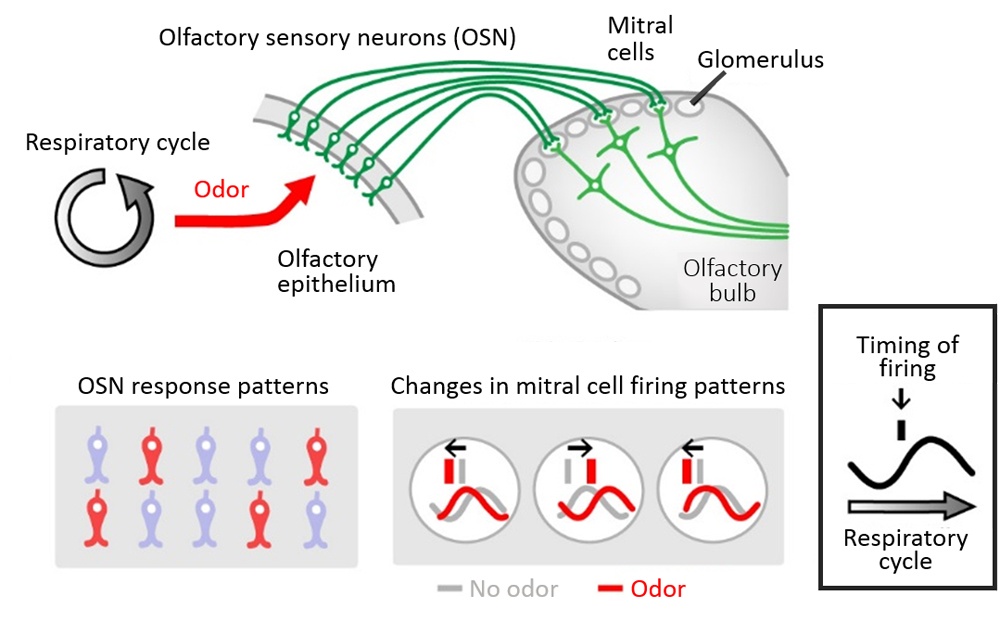
When sniffing or breathing in through the nose, odorants in the air are taken up into the nasal cavity where they bind to a specific OSNs located in the olfactory epithelium. The axons of OSNs extend to the glomeruli situated in the olfactory bulb of the brain and form synapses with dendritic spines of mitral cells. Odor stimuli transmitted from the OSNs, will excite mitral cells, which will in turn send information to the olfactory center to be interpreted as a specific odor. Past studies have demonstrated that mitral cells exhibited differences in firing rates and temporal firing patterns depending on the type of odor stimulus, and it was suggested that odor information was encoded by these two parameters. Recent studies have also shown that in addition to odor-evoked stimuli from OSNs, mitral cells also show responses to mechanical stimulation such as respiratory airflow in the nose. However, how mitral cells can differentiate between odor stimuli and mechanical stimuli remained unclear, as observing mitral cell activity in vivo was difficult. To try elucidating the mechanism involved in odor information processing, Iwata and his collaborators used two-photon calcium imaging, which facilitates deep tissue observations, and measured neuronal activity by looking at calcium dynamics in the olfactory bulb in response to odor stimuli.
The team first examined how OSNs in the olfactory epithelium detect mechanical signals. Their observations of the neural activity of the OSNs and the mitral cells in the glomerulus of the olfactory bulb revealed that when OSNs were stimulated by airflow, they showed robust and widespread responses, whereas downstream mitral cells showed oscillatory neuronal firing in response to the mechanical signals. Furthermore, the oscillation cycles varied between glomeruli in the olfactory bulb, and when airflow into the nasal cavity is physically blocked, no oscillatory activity was seen and instead neuronal firing was spontaneous and sporadic. These results suggested that airflow-derived mechanical signals that are transmitted by OSNs are required to produce the distinct oscillations of neural activity in mitral cells.
So what is the relationship between mitral cell firing responses triggered by mechanical signals, and those responses to odor-evoked signals? It was known mitral cells show changes in firing rates and temporal firing in response to odor stimulus, but which property was more important for discriminating odors was not well understood. The team compared and analyzed the mitral cell firing patterns when a mouse was continuously exposed to the same odor over multiple sniff cycles. They observed that temporal firing patterns of the mitral cell remained stable, while the firing rate changed dynamically. They also examined the firing patterns for different concentration of the odor, and again found that the temporal patterns are maintained regardless of odor concentration. Thus, the observations suggested that mitral cells encode odor “identity” information as temporal code. They also demonstrated that mechanosensory-driven oscillatory neural activity in mitral cells improves olfaction, acting as a pacemaker for temporal patterning, as evidenced by experiments showing reduced precision of mitral cell firing under continuous airflow.
“It has long been thought that odors are discriminated by the combination of odorants binding to OSNs. But our study demonstrated that the temporal firing pattern of mitral cells is critical for identifying odors. Further, mechanosensation by airflow creates oscillations of neural activity, which is important to ensure OSNs are activated at a precise timing during a sniff cycle,” explains Imai. “Our next goal is to understand the how the precise temporal neural firing patterns are generated.”
| Link to article |
Mechanosensory-Based Phase Coding of Odor Identity in the Olfactory Bulb |
|---|