The central question in developmental biology is how cells, tissues and organs acquire their specific functions and shapes. A large body of work over the past several decades has yielded a broad understanding of how functional specialization is achieved through differential gene expression. In contrast, far less is known about how cell shapes and tissue structures are controlled and remodeled. Although a general theme has emerged whereby cytoskeletal elements control the cell shapes, while alteration of individual cell shapes collectively organizes the tissue architecture, the underlying molecular and mechanical mechanisms remain poorly understood. My lab aims at identifying novel mechanisms that orchestrate the formation of three-dimensional epithelial structures. Our long-term goal is to comprehensively understand the mechanistic principles of tissue morphogenesis in order to conceptualize the origin of morphological diversity both within an organism and among evolutionary lineages.
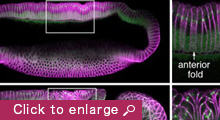
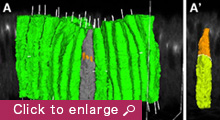
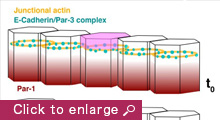
We are currently focusing on how modifications of epithelial cell polarity control cell shapes using gastrulating Drosophila embryos as the model system. Our previous work identified a novel mechanism for cell shape changes whereby cell shortening is induced upon a basal repositioning of the apical-basal polarity and cell-cell adhesive apparatus adherens junctions. The cell shortening occurs in two narrow strips of cells, producing heterogeneities in cell height within the tissue, thereby allowing it to bend. The polarity-based mechanism represents the first instance wherein the initiation of epithelial folding does not involve the canonical myosin-dependent apical constriction. Since cell-cell adhesion and apical-basal polarization are two fundamental features of epithelial tissues, our work potentially heralds a general mechanism for cell shape changes and epithelial folding. In addition, we found that after initiation, the depths of epithelial folds differ depending on the degrees of neighboring cell invagination. Genetic evidence suggests that the strength of mechanical coupling between adherens junctions and their underlying actin cytoskeleton determines the extent of cell invagination. Our ongoing work promises to identify genes and forces that sculpt distinct morphological features.
We employ an integrated approach that combines genetic manipulation, two-photon deep tissue live imaging and computational cell shape reconstruction. We are also in the process of designing novel imaging strategies that could be used to visualize mechanical forces and computational algorithms that reconstruct and quantify 4D cell shapes. Furthermore, we will launch a multidisciplinary, international collaboration that combines genetics, computational and evolutionary approaches to analyze the history and function of transiently formed epithelial structures that do not eventually contribute to a body part or organ.

A:We are passionate about making new and important discoveries in the filed of developmental biology. We come to work everyday with great enthusiasm knowing that we will understand better how cells undergo the necessary change to form a functional organism.
A:Dynamic. My lab is shaped and morphed by the unique personalities and posive energies of its indivisual members in an organic and creative way.
A:Let your own imagination be the only limitation, and the desire to know more be the preferred way to propel forward.
ycwang[at]cdb.riken.jp