
News and Announcements from the CDB
Age-related macular degeneration (AMD) is one of the most commonly diagnosed eye disorders causing impaired vision in older populations in developed countries. There are two known sub-types of AMD—neovascular (wet-type) AMD and atrophic (dry-type) AMD. Within Japan, wet-type AMD is the more prevalent condition, and is characterized by abnormal blood vessel formation (neovascularization) in an area beneath the center of the retina (macula) at the back of the eye, which damages and impedes function of the retinal pigment epithelium (RPE). The RPE is a monolayer sheet of cells lining the outer surface of the neural retina and plays multiple roles in the eye from absorbing light entering the eye, serving as a barrier between the eye and the choroid blood vessels, to nourishing photoreceptor cells and clearing away their metabolic waste. The gradually deteriorating function of the RPE in AMD patients leads to blurriness or blind spots in the center of their vision, and while it does not often result in complete blindness, it can significantly affect the patients’ quality of life.
In August 2013, a pilot clinical study was launched to examine the feasibility and safety of transplanting an RPE cell sheet generated from autologous (patient-derived) induced pluripotent stem cells (iPSCs) into AMD patients. The study was led by Project Leader Masayo Takahashi and Deputy Project Leader Michiko Mandai of the CDB’s Laboratory for Retinal Regeneration, and carried out in collaboration with the Institute for Biomedical Research and Innovation (IBRI) Hospital, Kobe Medical Center General Hospital, and Kyoto University’s Center for iPS Cell Research and Application (CiRA). The first iPSC-RPE cell sheet transplantation was carried out in September 2014 (Science News: September 15, 2014), and the team presented the details of their clinical study, including protocols, observations and outcomes, in a paper published in The New England Journal of Medicine.
Currently available treatments for AMD primarily target specific symptoms, such as periodic injections of anti-vascular endothelial growth factor (VEGF) drugs to suppress further blood vessel formation, which only delays disease progression and does not cure the disease itself. Many groups have been working to establish more effective methods to restore function of damaged RPE in AMD, including transplantation of sheets or cell suspensions of RPE cells derived from human fetuses or embryonic stem cells, which has shown mixed results, from some improvement to rejection of transplanted cells as well as adverse events associated with use of immunosuppressants. Research in CDB’s former Laboratory for Organogenesis and Neurogenesis led by the late Yoshiki Sasai had established methods to differentiate pluripotent stem cells into retinal cells, which was then refined by Takahashi’s group to generate retinal pigment epithelial cells. This laid the foundation to launch a clinical study examining the safety of transplanting RPE cell sheets made from autologous iPSCs into the patient’s eye, as well as look at the feasibility of using this approach to treat AMD.
Two patients diagnosed with wet-type AMD were enrolled to participate in the clinical study, the first patient was a woman in her 70s and the second patient, a man in his 60s. The team used non-integrating episomal vectors to generate iPSCs from skin fibroblast cells taken from each patient, which were then differentiated into RPE cells. These RPE cells were purified, cultured into a cell sheet, and tested for quality and safety against the standards outlined in the protocol, which included analyses of cell viability, morphology, immunostaining, assessments of RPE cell sheet function, and tests for tumorigenicity. DNA methylation and gene expression profiles were also analyzed as reference data. Following these tests, the team obtained two RPE cell lines from the first patient and one RPE cell line from the second patient that met the protocol criteria for transplantation.
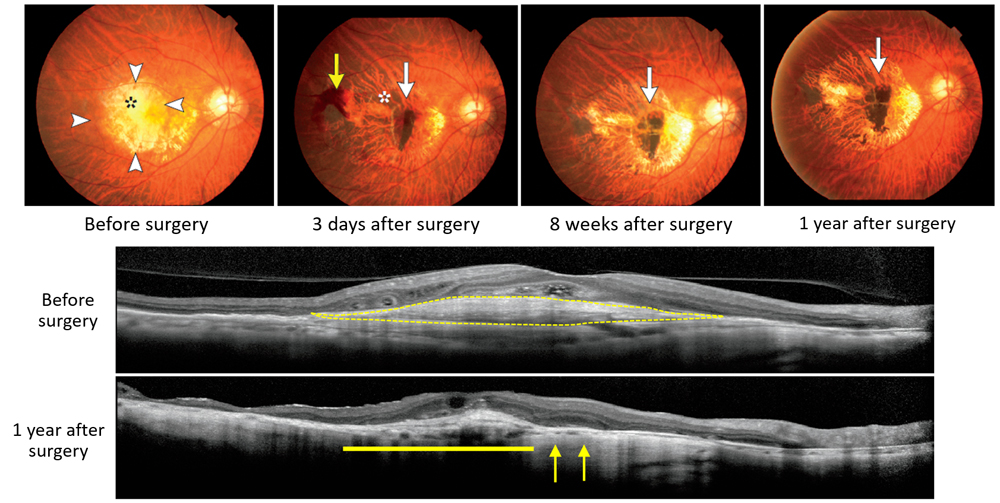
The first patient underwent transplant surgery on September 12, 2014, which involved removal of ectopically formed blood vessels and damaged RPE area, and then transplant of iPSC-derived RPE cell sheet (1.3X3.0 mm) under the retina. No serious adverse events were detected following the surgery, and changes that had resulted from abnormal blood vessel formation were resolved within a short period after the surgery. To date, the transplanted RPE cell sheet has remained intact in the originally transplanted area, and no signs of rejection or tumor formation have been observed. The patient’s vision which had been deteriorating despite regular anti-VEGF injections prior to the transplant, appears to have stabilized, showing neither significant improvement or worsening after the surgery.
The RPE cells generated from the second patient also passed required quality and safety tests, including that for tumorigenicity, however, whole-genome sequencing analyses revealed some genetic changes had occurred during the derivation of iPSCs and iPSC-derived RPE cells lines. Because there were no criteria to evaluate how these changes could affect patient, as well as the fact that his symptoms had stabilized with anti-VEGF injections, the team decided not to carry out the surgery in the second patient.
“The results of this clinical study indicate that the use of autologous iPSCs as a source for cell-based therapy in AMD is safe and feasible. If ongoing studies validate safety of the approach, earlier stage interventions may stop progression or even improve visual acuity of AMD patients,” explains Takahashi. “Concerns associated with autologous iPSC such as long preparation times and high costs still need to be addressed, and a shared consensus is needed among scientists and clinicians regarding acceptable quality and safety standards of iPSC-derived cells used in transplantations. We hope to clear each of these hurdles, moving closer to achieving our goal of establishing iPSC-based therapies for AMD and other retinal diseases.”
| Link to article |
Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. |
|---|---|
| Related links |
Transplantation of iPSC-derived RPE sheet into first AMD patient |